Zero First Second Order Graphs
Zero First Second Order Graphs - Web Sep 12 2022 nbsp 0183 32 Since the first order plot left is not linear we know that the reaction is not first order The linear trend in the second order plot right indicates that the reaction follows second order kinetics Two graphs are shown each Web Feb 13 2023 nbsp 0183 32 You learned that the integrated rate law for each common type of reaction zeroth first or second order in a single reactant can be plotted as a straight line Using these plots offers an alternative to the methods described for showing how reactant concentration changes with time and determining reaction order Web To use graphs to analyze the kinetics of a reaction In Section 14 3 quot Methods of Determining Reaction Order quot you learned that the integrated rate law for each common type of reaction zeroth first or second order in a single reactant can be plotted as a straight line
If ever you are looking for a efficient and simple method to improve your performance, look no further than printable templates. These time-saving tools are free and easy to use, offering a variety of benefits that can help you get more carried out in less time.
Zero First Second Order Graphs

First order Reaction Definition Examples And Equations
 First order Reaction Definition Examples And Equations
First order Reaction Definition Examples And Equations
Zero First Second Order Graphs Printable design templates can help you remain arranged. By supplying a clear structure for your tasks, to-do lists, and schedules, printable design templates make it simpler to keep everything in order. You'll never ever have to stress over missing out on deadlines or forgetting crucial jobs once again. Utilizing printable templates can help you conserve time. By removing the need to develop brand-new documents from scratch whenever you require to complete a task or prepare an occasion, you can focus on the work itself, rather than the documents. Plus, numerous design templates are personalized, allowing you to individualize them to fit your requirements. In addition to saving time and staying arranged, utilizing printable design templates can also help you stay motivated. Seeing your progress on paper can be an effective incentive, encouraging you to keep working towards your objectives even when things get tough. In general, printable design templates are a great way to boost your productivity without breaking the bank. So why not give them a shot today and start achieving more in less time?
IB Chem Helper 16 Kinetics HL
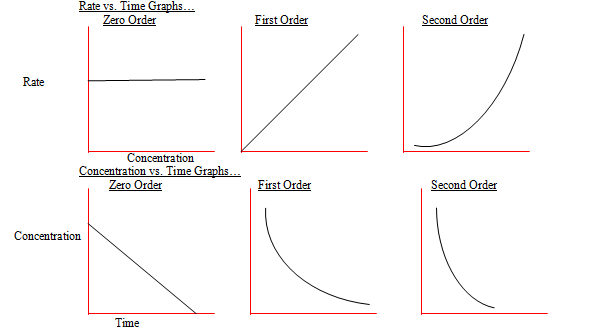 Ib chem helper 16 kinetics hl
Ib chem helper 16 kinetics hl
Web Apr 12 2023 nbsp 0183 32 A plot of ln NO 2 versus t part b in Figure 14 4 1 shows us that the reaction is not first order in NO 2 because a first order reaction would give a straight line Having eliminated zeroth order and first order behavior we construct a plot of 1 NO 2 versus t part c in Figure 14 4 1
Web However the half life of a zero order reaction increases as the initial concentration increases Equations for both differential and integrated rate laws and the corresponding half lives for zero first and second order reactions are summarized in Table 12 2
Using Graphs To Determine Rate Laws Rate Constants And Reaction Orders
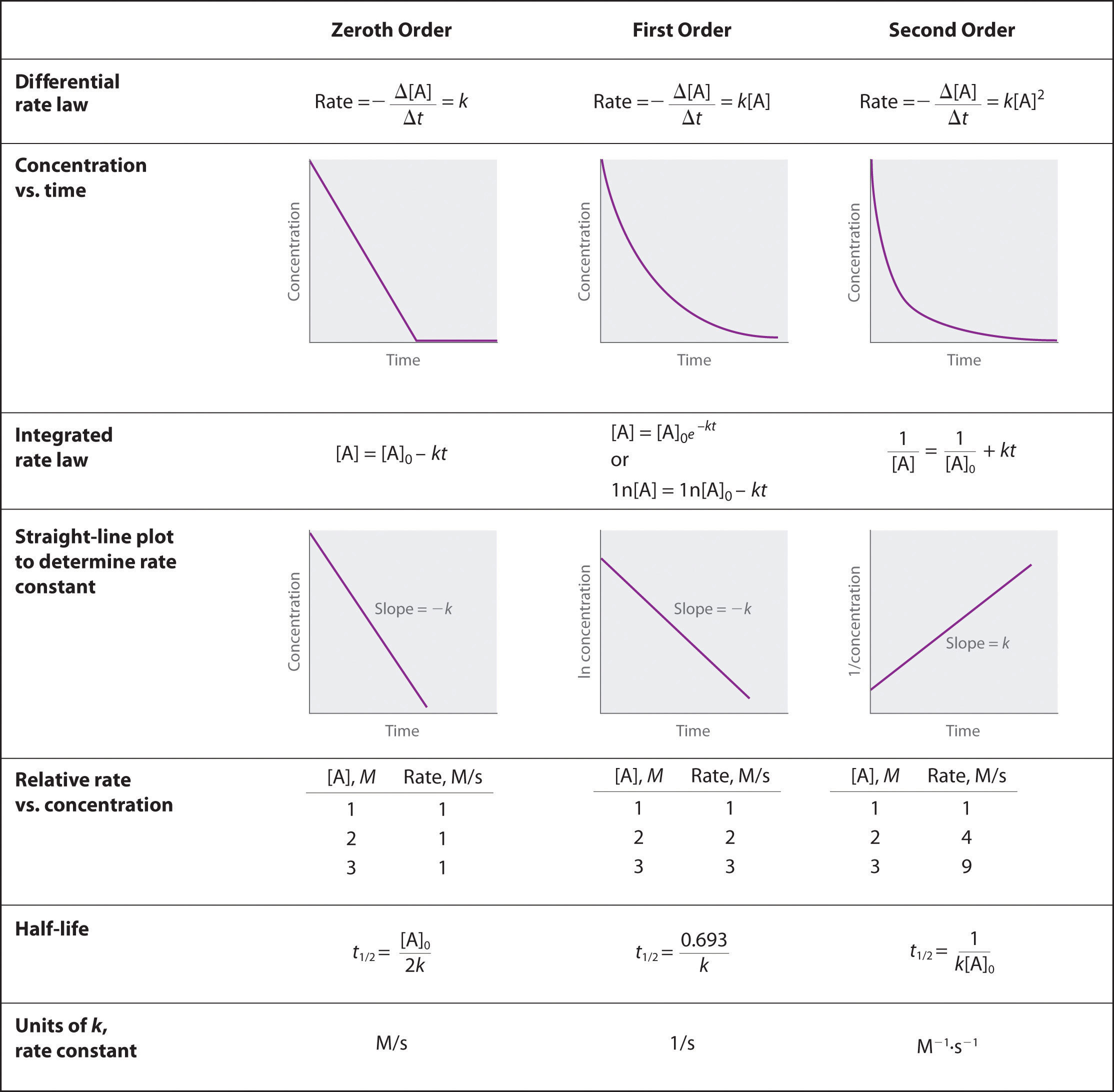 Using graphs to determine rate laws rate constants and reaction orders
Using graphs to determine rate laws rate constants and reaction orders
Law On Pinterest
 Law on pinterest
Law on pinterest
Free printable design templates can be an effective tool for increasing efficiency and attaining your goals. By choosing the ideal design templates, incorporating them into your regimen, and personalizing them as needed, you can streamline your everyday tasks and take advantage of your time. Why not give it a try and see how it works for you?
Web The graph that is linear indicates the order of the reaction with respect to A Then you can choose the correct rate equation For a zero order reaction rate k k slope of line order reaction rate k A k slope of line order reaction rate k A k slope of line
Web Feb 13 2023 nbsp 0183 32 The graph of a zeroth order reaction The change in concentration of reactant and product with time produces a straight line The integrated rate law for a zeroth order reaction also produces a straight line and has the general form A A 0 kt 14 16 14 16 A A 0 k t