Intensive And Extensive Properties Examples
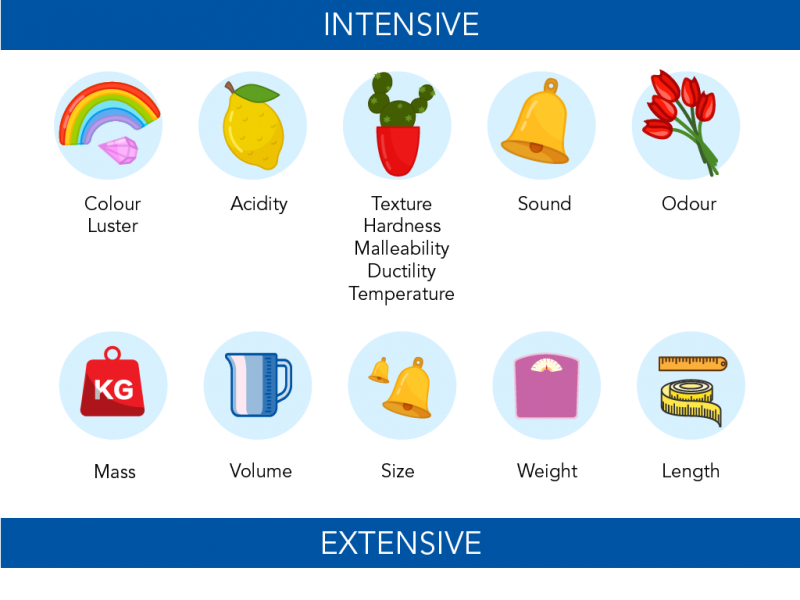
Intensive And Extensive Properties Examples - Examples of intensive properties Examples of extensive properties Examples of extensive properties Examples of intensive properties Temperature The amount of heat in a substance It is measured in degrees For example This water sample has a temperature of 32 degrees Celsius Volume energy and mass are examples of extensive properties Extensive Property Examples There are properties such as length mass volume weight etc that depend on the quantity or size of the matter these properties are called an extensive property of matter and their value changes if the size or quantity of matter changes The ratio of any two extensive properties is an intensive property The most common example is density which is the ratio of mass and volume both extensive but is itself intensive since it does not change as the amount of a substance changes rho dfrac m V V m
Look no even more than printable templates whenever you are looking for a easy and effective method to enhance your productivity. These time-saving tools are easy and free to utilize, providing a variety of benefits that can help you get more done in less time.
Intensive And Extensive Properties Examples

Difference Between Intensive And Extensive Properties Of Matter Properties Of Matter Intense
 Difference Between Intensive And Extensive Properties Of Matter Properties Of Matter Intense
Difference Between Intensive And Extensive Properties Of Matter Properties Of Matter Intense
Intensive And Extensive Properties Examples Printable templates can assist you stay arranged. By supplying a clear structure for your jobs, to-do lists, and schedules, printable templates make it simpler to keep everything in order. You'll never ever have to stress over missing due dates or forgetting crucial jobs once again. Secondly, utilizing printable design templates can assist you conserve time. By eliminating the need to develop new documents from scratch whenever you need to complete a task or plan an occasion, you can concentrate on the work itself, rather than the documentation. Plus, lots of templates are customizable, permitting you to personalize them to match your requirements. In addition to saving time and remaining arranged, using printable templates can also help you remain encouraged. Seeing your development on paper can be an effective motivator, encouraging you to keep working towards your goals even when things get hard. Overall, printable templates are a terrific way to increase your efficiency without breaking the bank. So why not give them a try today and start attaining more in less time?
Intensive Vs Extensive Properties with Examples PSIBERG
Intensive vs extensive properties with examples psiberg
Examples of intensive properties include temperature T refractive index n density and hardness By contrast an extensive property or extensive quantity is one whose magnitude is additive for subsystems 4 Examples include mass volume and entropy 5
Examples include density state of matter and temperature Extensive properties do depend on sample size Examples include volume mass and size Intensive Properties Intensive properties are bulk properties which means they do not depend on the amount of matter that is present Examples of intensive properties include Boiling Point Density
How Do They Use Atoms To Explain Different Physical Properties
 How do they use atoms to explain different physical properties
How do they use atoms to explain different physical properties
An Intensive Property Of A Substance Is Property Walls
An intensive property of a substance is property walls
Free printable templates can be an effective tool for boosting efficiency and achieving your objectives. By selecting the ideal templates, including them into your regimen, and customizing them as needed, you can simplify your everyday jobs and make the most of your time. So why not give it a try and see how it works for you?
Extensive properties vary with the amount of the substance and include mass weight and volume Intensive properties in contrast do not depend on the amount of the substance they include color melting point boiling point electrical conductivity and physical state at a given temperature For example elemental sulfur is a yellow
For example pure copper is always a reddish brown solid a physical property and always dissolves in dilute nitric acid to produce a blue solution and a brown gas a chemical property Physical properties can be extensive or intensive Extensive properties vary with the amount of the substance and include mass weight and volume