What Is An Isotope
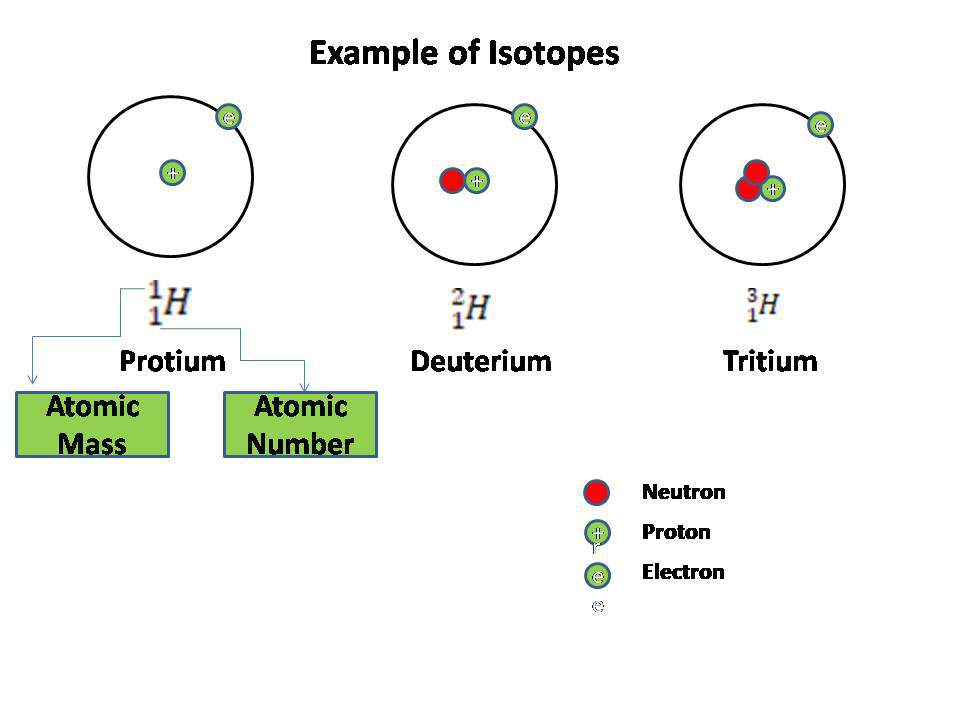
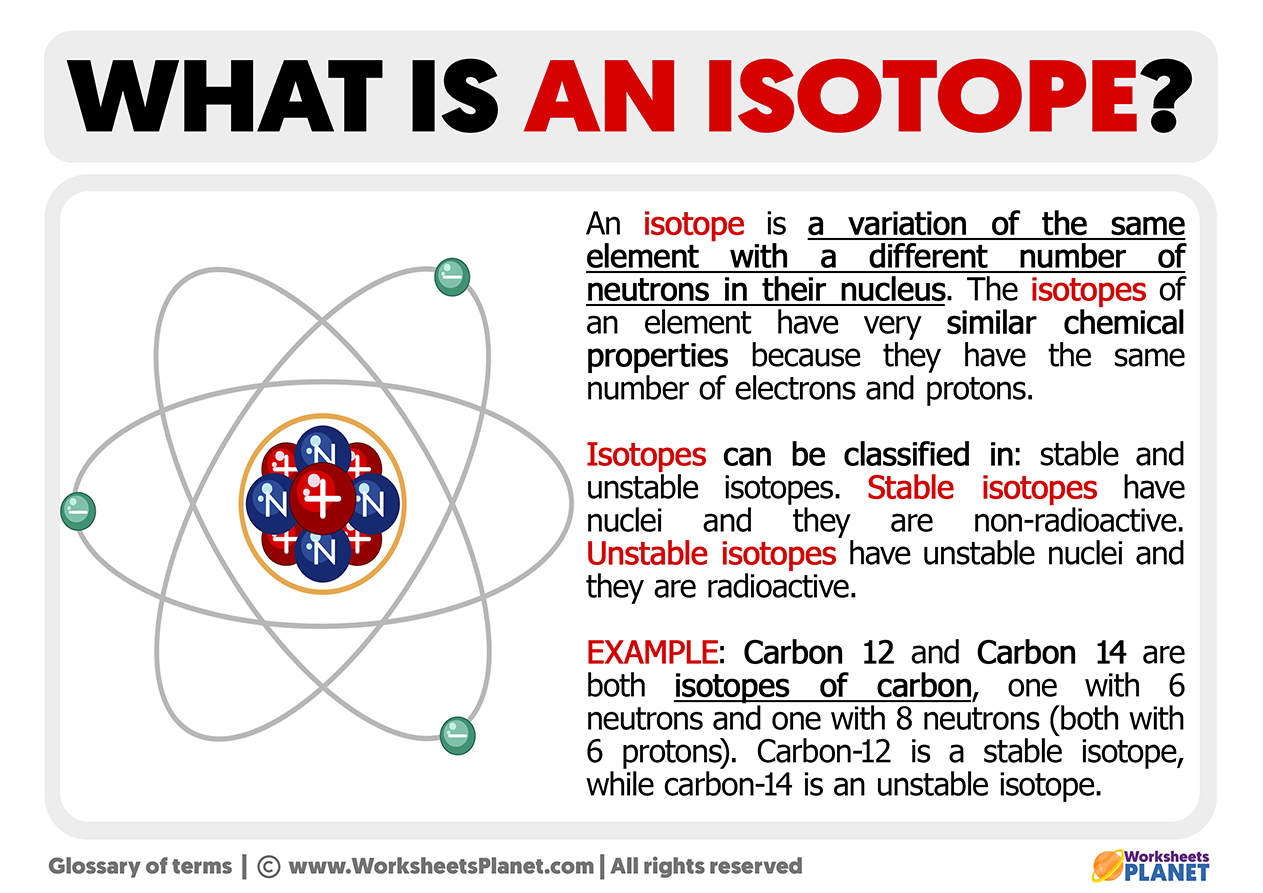
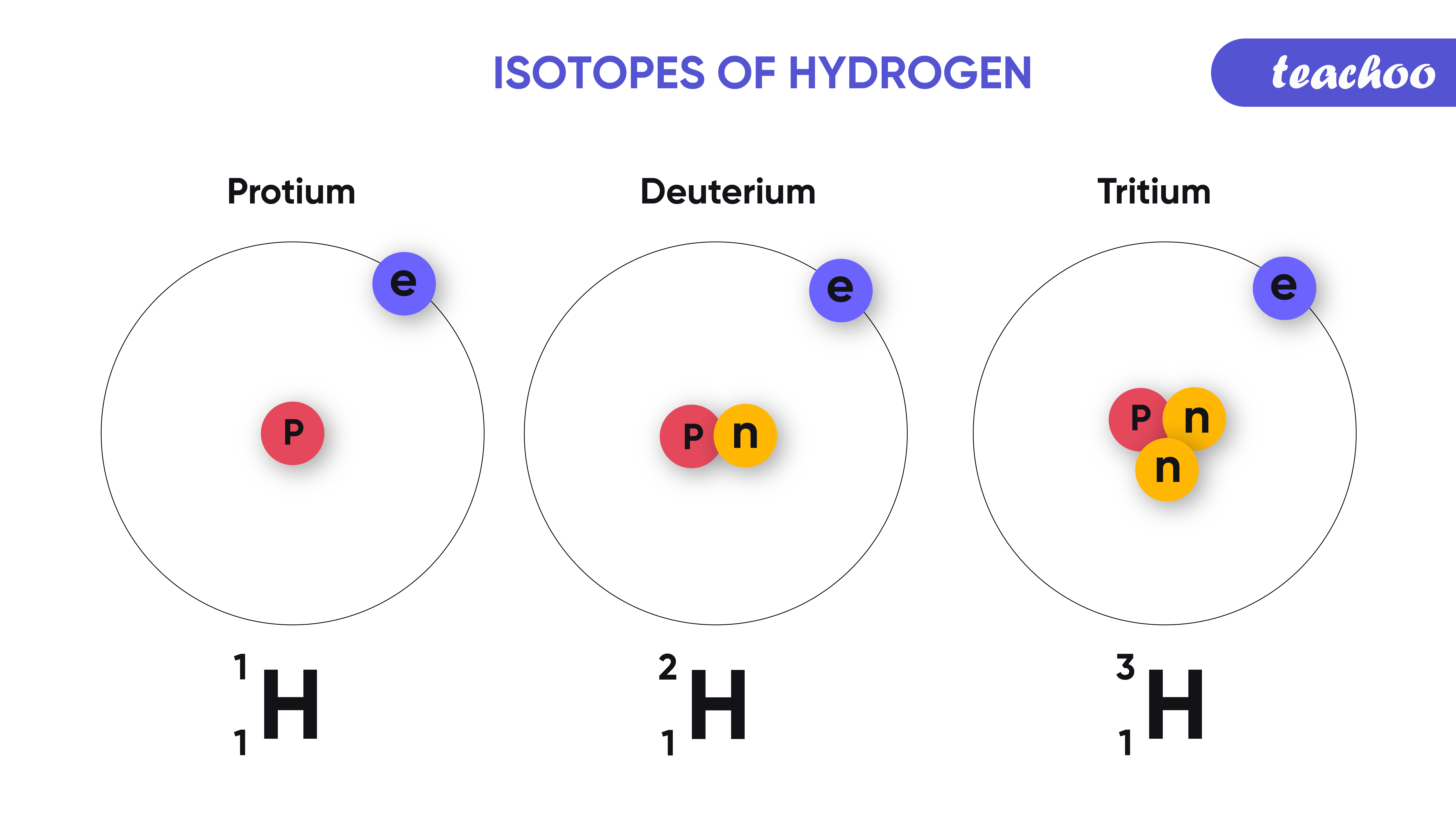
What Is An Isotope - The term isotopes originally also isotopic elements now sometimes isotopic nuclides is intended to imply comparison like synonyms or isomers For example the nuclides 12 6 C 13 6 C 14 6 C are isotopes nuclides with the same atomic number but different mass numbers but 40 18 Ar 40 19 K 40 20 Ca are isobars nuclides with the same An isotope is a variation of an element that possesses the same atomic number but a different mass number A group of isotopes of any element will always have the same number of protons and electrons They will differ in the number of neutrons held by their respective nuclei Atoms that have the same atomic number number of protons but different mass numbers number of protons and neutrons are called isotopes There are naturally occurring isotopes and isotopes that are artificially produced
In case that you are trying to find a easy and efficient way to improve your productivity, look no further than printable design templates. These time-saving tools are easy and free to use, providing a variety of advantages that can assist you get more done in less time.
What Is An Isotope
What Is An Isotope Definition Of Isotope
 What Is An Isotope Definition Of Isotope
What Is An Isotope Definition Of Isotope
What Is An Isotope Firstly, printable templates can assist you remain arranged. By supplying a clear structure for your jobs, order of business, and schedules, printable design templates make it simpler to keep whatever in order. You'll never have to fret about missing due dates or forgetting important tasks once again. Secondly, utilizing printable templates can assist you save time. By eliminating the requirement to create new documents from scratch whenever you require to finish a job or prepare an event, you can focus on the work itself, instead of the documents. Plus, numerous templates are personalized, permitting you to personalize them to match your requirements. In addition to conserving time and staying organized, utilizing printable design templates can also help you remain motivated. Seeing your development on paper can be a powerful motivator, encouraging you to keep working towards your objectives even when things get tough. In general, printable design templates are an excellent way to improve your performance without breaking the bank. Why not offer them a shot today and begin accomplishing more in less time?
Isotopes Definition And Examples In Chemistry
/Isotope-58dd6b415f9b5846830254ae.jpg) Isotopes definition and examples in chemistry
Isotopes definition and examples in chemistry
Isotopes are samples of an element with different numbers of neutrons in their atoms The number of protons for different isotopes of an element does not change Not all isotopes are radioactive Stable isotopes either never decay or else decay very slowly Radioactive isotopes undergo decay
The meaning of ISOTOPE is any of two or more species of atoms of a chemical element with the same atomic number and nearly identical chemical behavior but with differing atomic mass or mass number and different physical properties How
Isotopes And Isobars Definition Uses And Difference Teachoo
 Isotopes and isobars definition uses and difference teachoo
Isotopes and isobars definition uses and difference teachoo
Isotopes By Rosi Miranda
 Isotopes by rosi miranda
Isotopes by rosi miranda
Free printable templates can be a powerful tool for improving performance and attaining your objectives. By picking the best templates, incorporating them into your routine, and personalizing them as required, you can improve your day-to-day tasks and take advantage of your time. So why not give it a try and see how it works for you?
Like everything we see in the world isotopes are a type of atom the smallest unit of matter that retains all the chemical properties of an element Isotopes are forms of a chemical element with specific properties You can see the different chemical elements on the periodic table Graphic A Vargas IAEA
Isotope Basics What are Isotopes Atoms are composed of a cloud of electrons surrounding a dense nucleus that is 100 000 times smaller and comprised of protons and neutrons The number of protons i e atomic number Z determines the element for example a strontium nucleus always has 38 protons and a rubidium nucleus always has